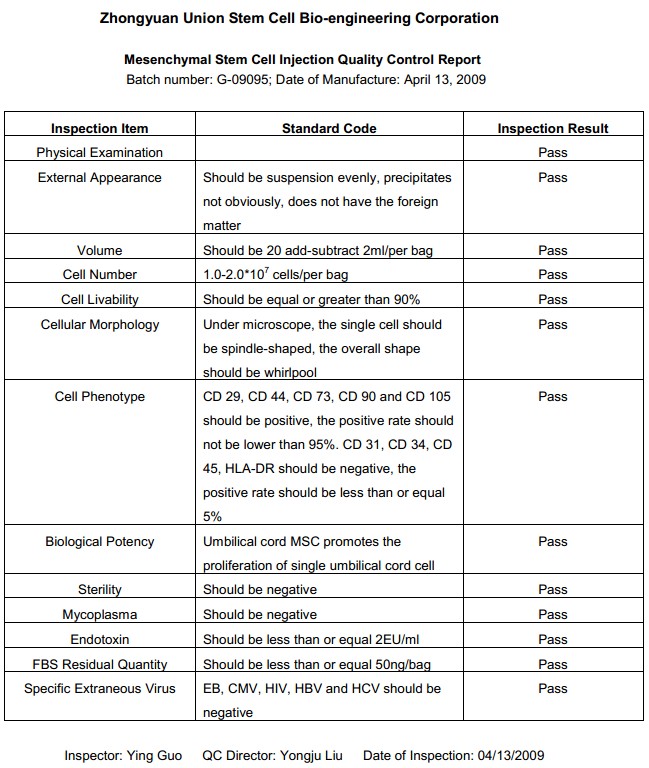
The Stem Cells We Use
To comply with the NICPBP (National Institute for the Control of Pharmaceutical & Biological Products, SFDA) standards for clinically used stem cells, the stem cells we use for transplantation are all processed and produced in the SFDA certified GMP facilities and are inspected through our "Quality-Certificate-Approval System." This ensures the highest product quality and safety.

The following QC report is from one of our stem cell providers "Zhongyuan Union Stem Cell Bio-engineering Corporation " which is China's largest stem cell research and manufacturing company (also known as "National Industrial Base of Stem Cell Technology" ) located in